Platform technology
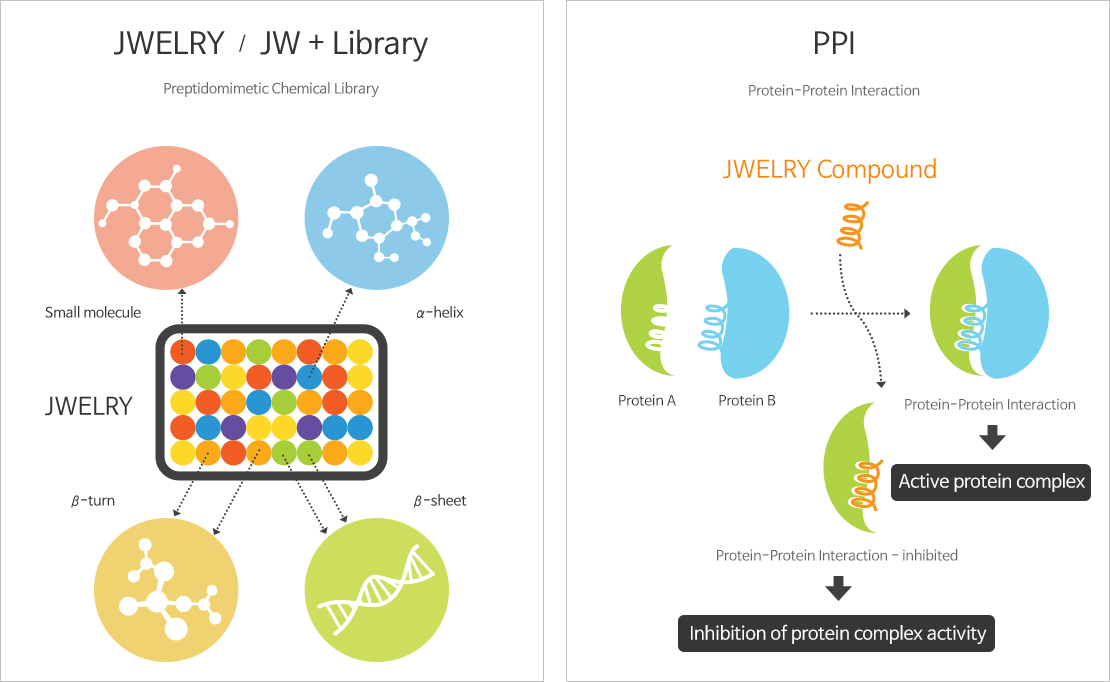
JWELRY (JW Excellent LibraRY)
- Compound library composed of unique peptide mimetic compounds
- A unique Drug Discovery Platform composed of diverse cancer cell lines and their genetic information.
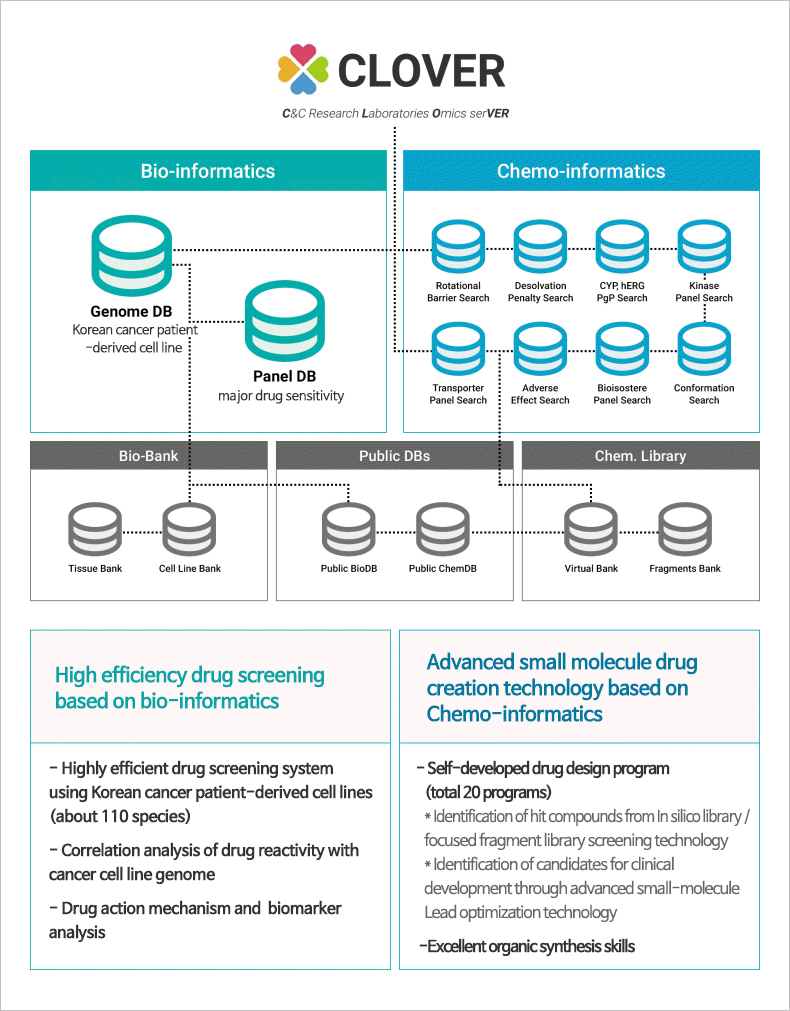
CLOVER (C&C research Laboratories Omics serVER)
Big data platform accumulating data of cancer cell lines, tissues, and genetic information, etc.
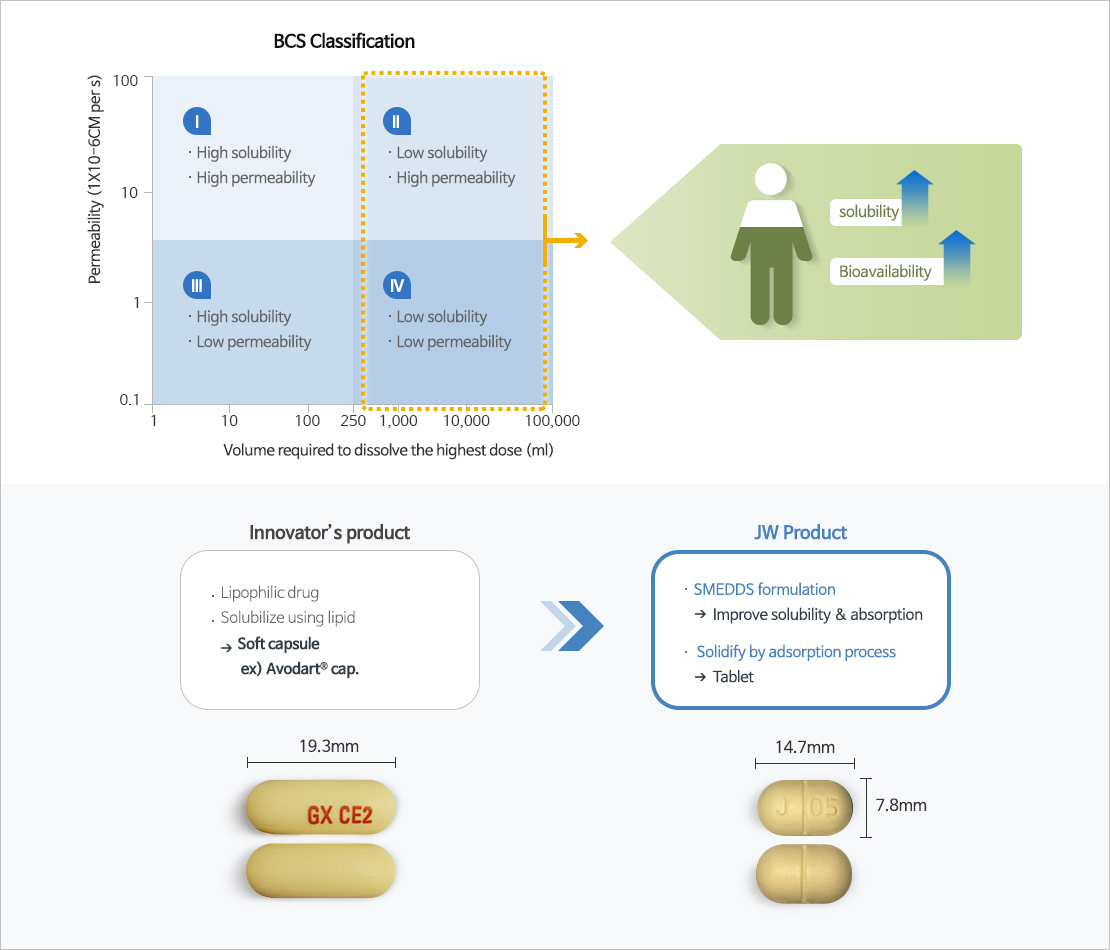
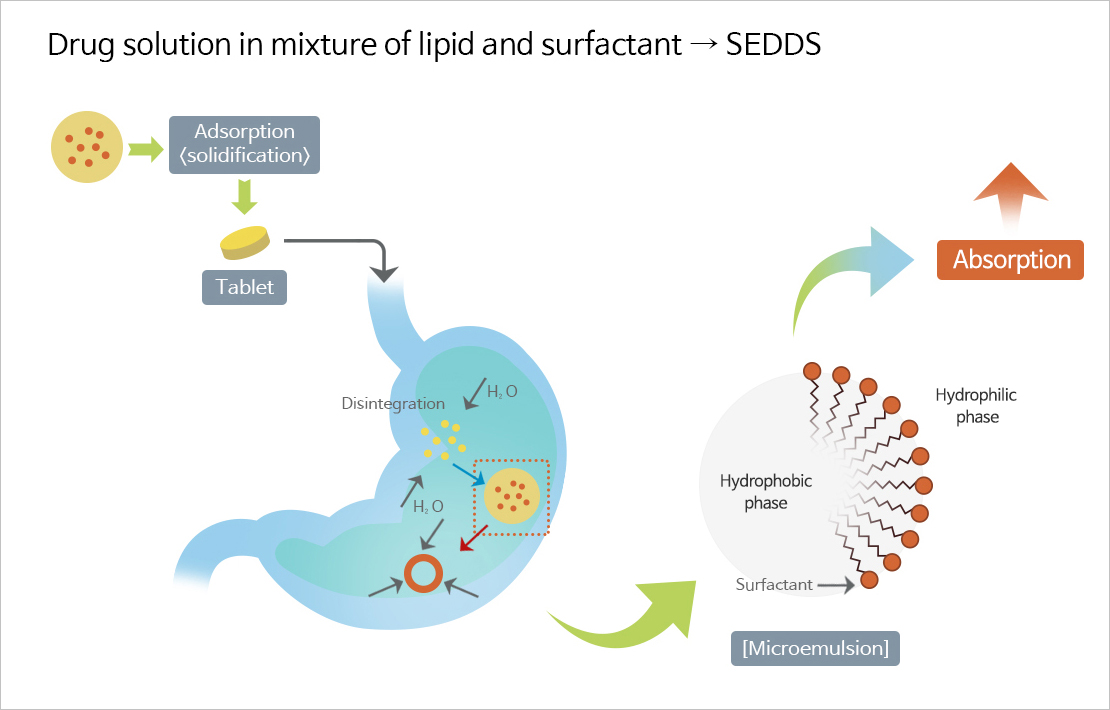
SMEDDS
- Self-microemulsifying drug delivery system (SMEDDS), one of the solubilizing technologies for poorly soluble drugs, is a technology that improves the solubility and bioavailability of poorly soluble drugs by mixing surfactant, co-surfactant, oil, and the drug at the optimum ratio.
- JW Pharmaceutical's proprietary technology, which overcomes the shortcomings and limitations of existing poorly soluble drug formulations and improves the solubility and bioavailability of drugs to maximize drug safety and ease of administration.
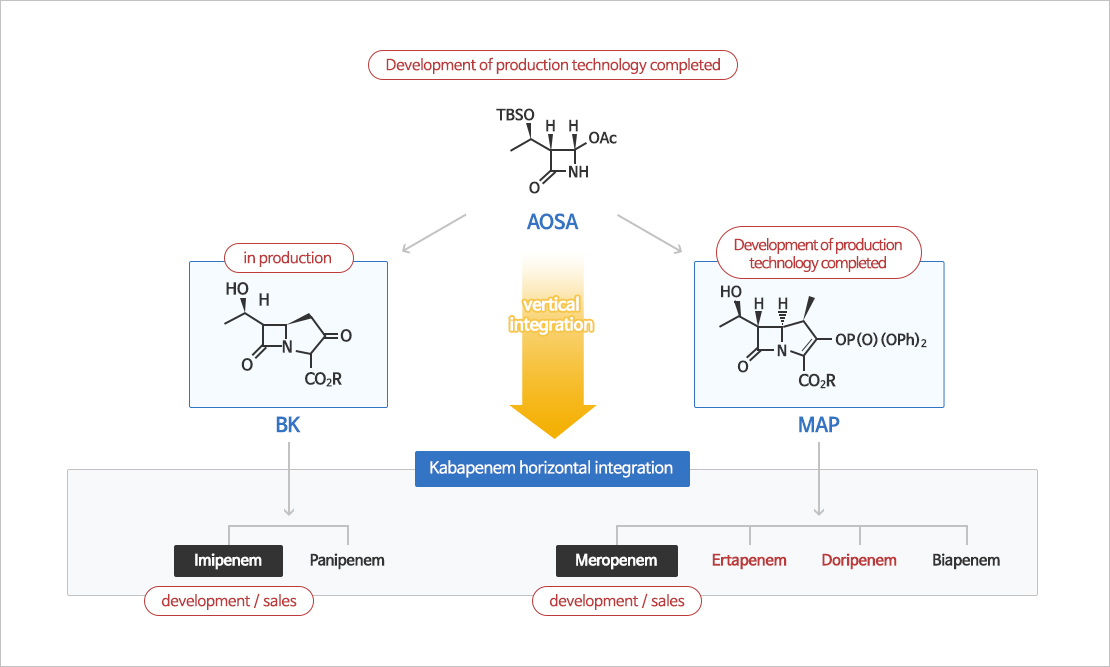
Carbapenem Manufacturing & Purification
Imipenem, meropenem and doripenem have been commercialized through a study of systematic using AOSA, a key starting material of carbapenems antibiotic agents.
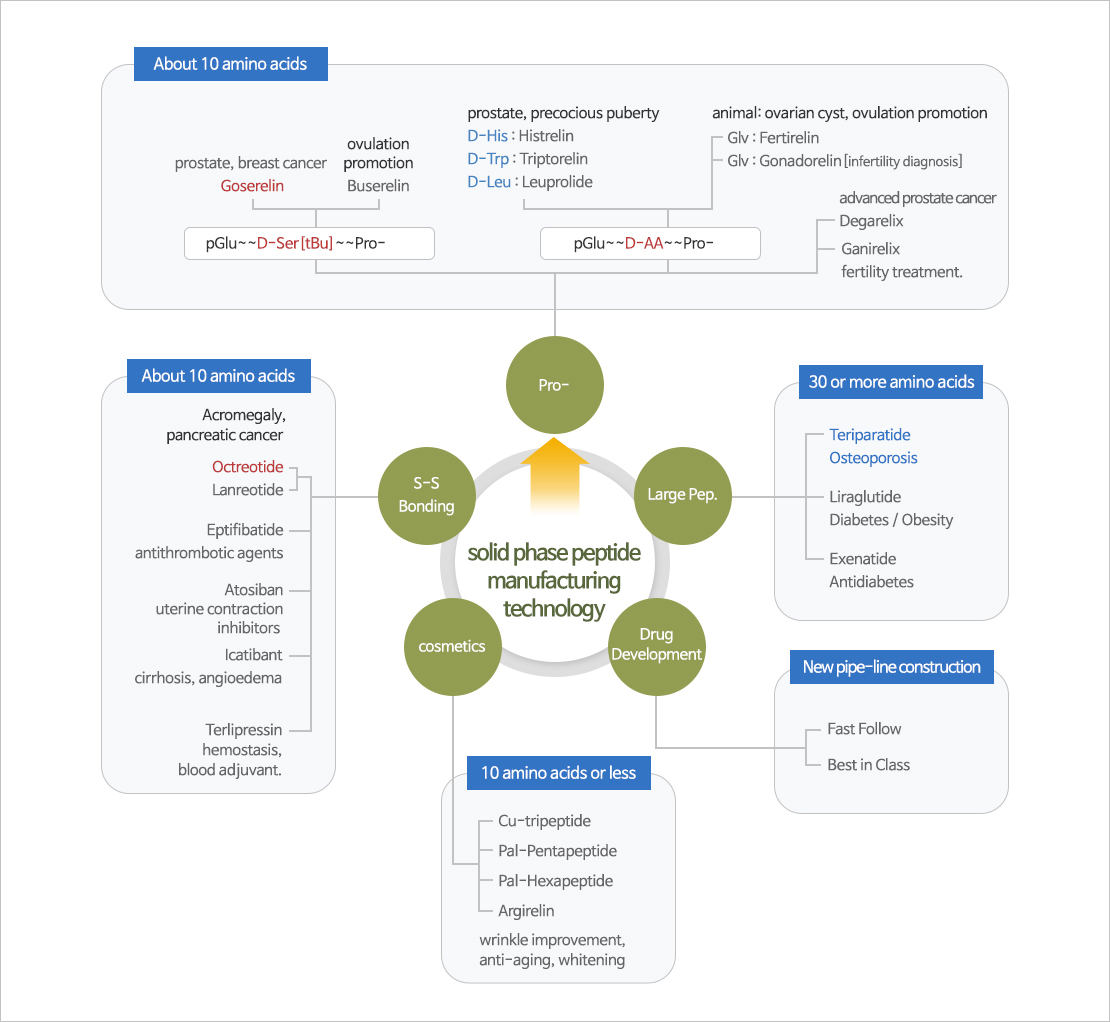
SPPS (Solid Phase Peptide Synthesis)
Manufacturing Process was completed through SPPS(solid phase peptide synthesis) and Prep-HPLC/Resin purification technology about Goserelin and Octreotide.
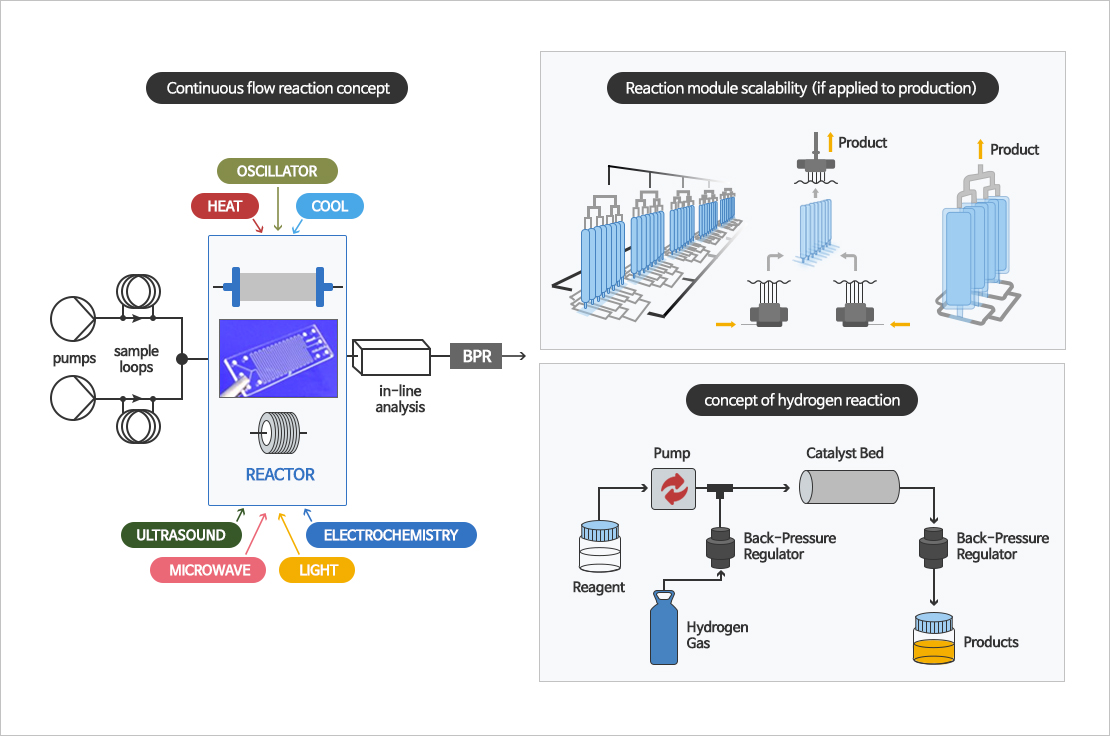
Continuous reaction, Flow chemistry reaction
Flow chemistry or continuous flow chemistry begins with two or more streams of different reactants pumped at specific flow rates into a microreactor or packed bed column. After reaction was complted, the reaction mixture is collected at the outlet. Because of the inherent design of specific module, hazardous and explosive reaction are possible than batch reaction. It can continuously manufacture high quality API.
-
R&D
Information
▼ - Research
Policy - Research
Center - Platform Technology
- Pipeline
- Research Network